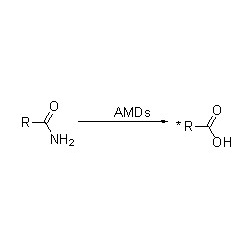
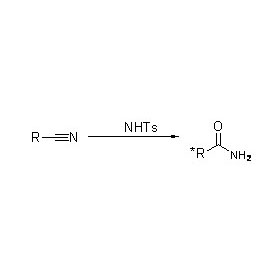
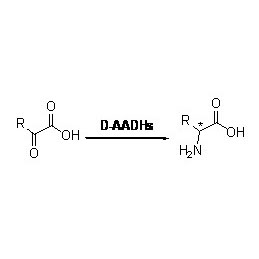
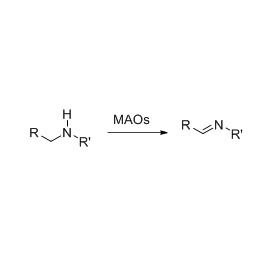
Amidase (AMD)
Enzymes:Sunt catalystae macromoleculares biologicae, maxime enzymes sunt proteins
Amidase:Hydrolysin variarum endogenarum et peregrinarum aliphaticorum et aromaticorum in medios catalyse, coetus acyl in aquam transferendo cum productione liberorum acida et ammoniaci.Acida hydroxamica et alia acida organica late pro medicamentis utuntur quia sunt factores incrementi, antibiotici et tumores inhibitores.Amidases dividi possunt in genus R et S genus acylases secundum catalyst stereoselectivity.
Praeter catalysin hydrolysis amides, etiam amidase acyl transferre reactiones coram co-substratis sicut hydroxylaminis catalyzis potest.
Amidase cum diversis fontibus diversam specificitatem substratam habent, quidam eorum solum hydrolyzare possunt amids aromaticis, quidam eorum solum hydrolyzare inter amicos aliphaticos, quidam hydrolyzare α-vel ω-amino amids.Plerique aminae bonam habent actionem catalyticam tantum pro acyclicis vel simplicibus aromatibus amids, sed pro aromatibus multiplicibus, amids heterocyclicis, praesertim amids ortho substituentibus, plerumque humiles sunt in actu (pauci tantum enzymes meliores effectus catalyticos exhibent).
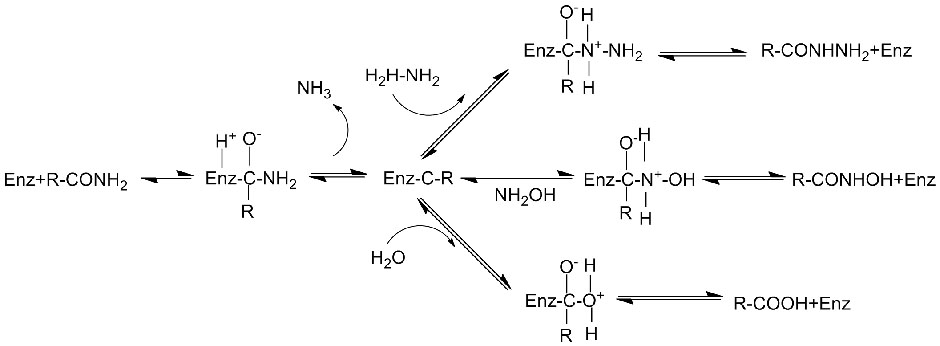
Mechanismus catalyticus:
| Enzymes | Product Code | Product Code |
| Enzyme Pulvis | ES-AMD-101~ ES-AMD-119 | a paro of 19 amidases, 50 mg inter 19 items * 50mg / item, vel alia quantitas |
| Ornamentum protegendo (SynKit) | ES-AMD-1900 | a paro of 19 amidases, 1 mg inter 19 items * 1mg / item |
Substantiae Substantiae High.
Chiral selectivity strong.
Maximum conversionem efficientiam.
★ Minus per-producta.
★ Mitis reactionis condiciones.
★ Environmentally amica.
Enzyme protegendo exerceri debet pro certis subiectis propter specificitatem subiecti, et enzyme quod catalyticum scopo substrati cum effectu catalytico optimo.
➢ Numquam attingunt extremas condiciones ut: caliditas, alta/humilis pH et organica solvendo cum concentratione alta.
➢ Plerumque, reactionem systema substratum, quiddam solutionem includere debet (optimam reactionem pH enzyme).Co-subiecta qualia hydroxylaminum praesentia esse debet in systematis reactionis translationis acyl.
➢ AMD addi debet ultimum in systematis reactionis cum optimam reactionem pH et temperie.
➢ Omnia AMD genera varias condiciones reactionis optimas habent, ut singulae singulae ulterius investigandae sint.
Exemplum 1(1):
Hydrolysis operatio diversae Amide Substrates
| Substratum | Imprimis actio μmols min-1mg-1 | Substratum | Imprimis actio μmols min-1mg-1 |
| Acetamide | 3.8 | ο-OH benzamide | 1.4 |
| Propionamide | 3.9 | p-OH benzamide | 1.2 |
| Lactamide | 12.8 | ο-NH2benzamide | 1.0 |
| Butyramide | 11.9 | p-NH2benzamide | 0.8 |
| Isobutyramide | 26.2 | ο-Toluamide | 0.3 |
| Pentanamide | 22.0 | p-Toluamide | 8.1 |
| Hexanamide | 6.4 | Nicotinamide | 1.7 |
| cyclohexanamide | 19.5 | Isonicotinamide | 1.8 |
| Acrylamide | 10.2 | Picolinamide | 2.1 |
| Metacrylamide | 3.5 | 3-Phenylpropionamide | 7.6 |
| Prolinamide | 3.4 | Indol-3-acetamide | 1.9 |
| Benzamide | 6.8 |
Reactio peracta est in 50mM sodium solutionis quiddam phosphate, pH 7.5, ad 70 .
| Amides | Hydroxylamine | Hydrazine |
| Acetamide | 8.4 | 1.4 |
| Propionamide | 18.4 | 3.0 |
| Isobutyramide | 25.0 | 22.7 |
| Benzamide | 9.2 | 6.1 |
Reactio peracta est in 50mM sodium solutionis quiddam phosphate, pH 7.5, ad 70 .
Concentratio reagentis: amid, 100 mM (benzamide, 10 mM);hydroxylamine et hydrazino, 400 mM;enzyme 0.9 μM.
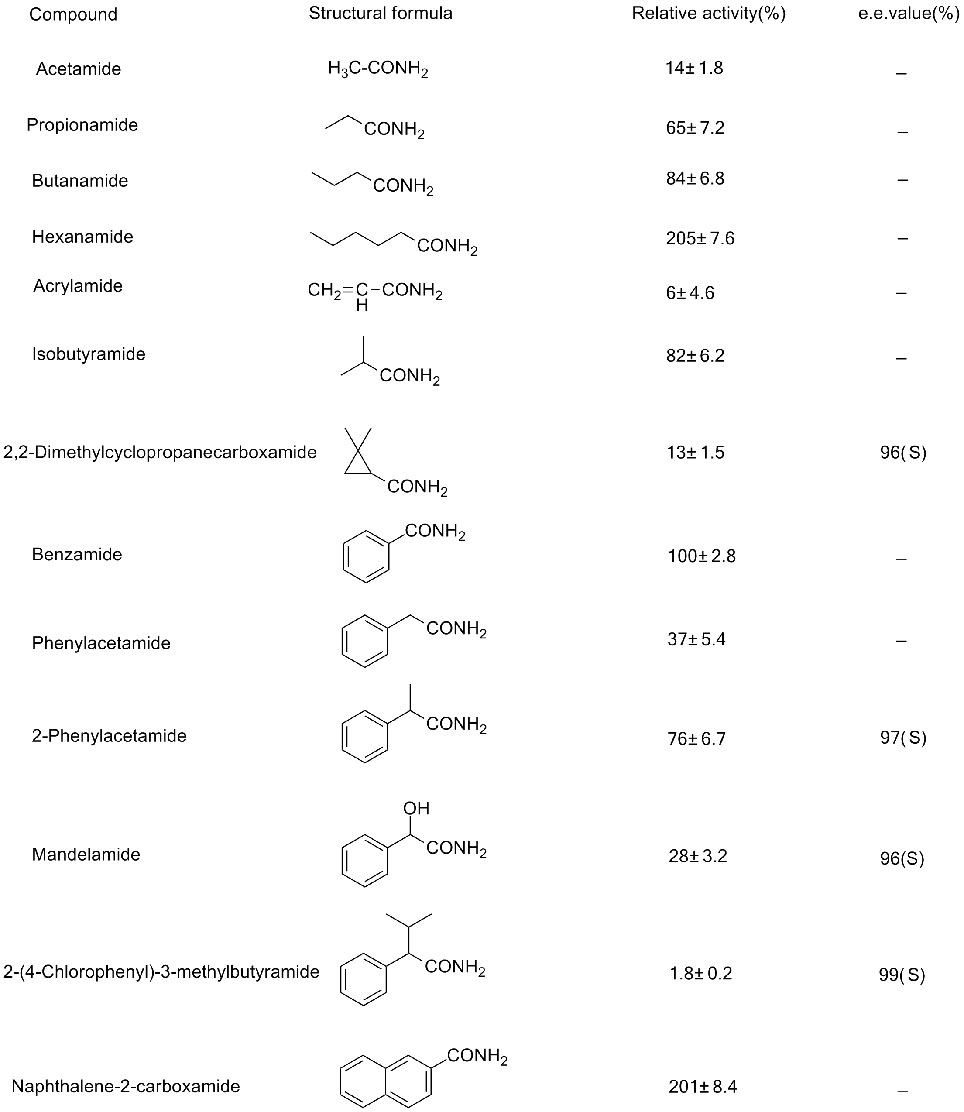
Exemplum 2(2):
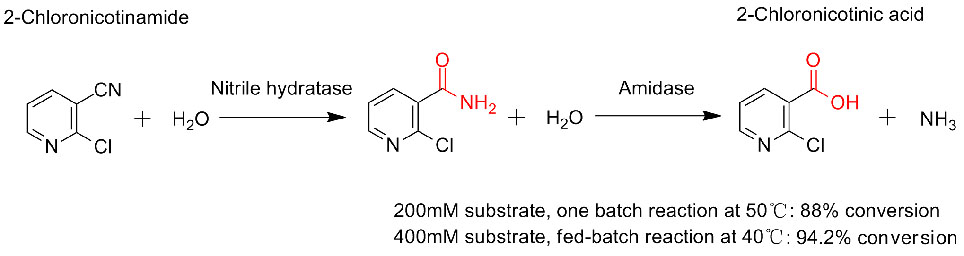
Exemplum 3(3):
1. D'Abusco AS, Ammendola S., et al.Extremophiles, 2001, 5:183-192.
2. Guo FM, Wu JP, Yang LR, et al.Processus Biochemistry, 2015, 50 (8): 1400-1404.
Zheng RC, Jin JQ, Wu ZM, et al.Chemistry Bioorganic, 2017, Available online.